

Botulinum Neurotoxin
Botulinum neurotoxin (BoNT) is a bacterial protein toxin with a molecular weight of 150 kDa produced by the anaerobic bacteria Clostridium botulinum, C. butyricum and C. baratii. BoNT comprise a
family of currently 32 isoforms which are divided into the seven serologically different groups BoNT/A-G. Lethal amounts in the range of ng per kg body weight constitute BoNT as
the most poisonous toxin.
Botulism
BoNT can cause several forms of the disease botulism, a flaccid paralysis of the muscle leading to death within a few days
due to respiratory failure. The classical form named food borne botulism is the oral intoxication by consumption of contaminated food. Here, the BoNT has to be shielded by a
non-toxic clostridial protein called NTNHA to survive the passage through the stomach and intestine (Gu et al., 2012). Hemagglutinins in a large
complex with BoNT and NTNHA (Lee et al., 2013) facilitate intestinal
absorption by disrupting the epithelial barrier (Lee et al., 2014) so that BoNT reaches the
circulation (Matsumura et al., 2015). In case of infant botulism the gut of newborns (up to 12 months age)
is colonised with C. botulinum and the BoNT is released at its site of resorption. Wound botulism is caused by infection of wounds with the ubiquitous spores of C. botulinum
analogous to the disease tetanus. Once the BoNT has reached the circulation it binds highly specific to cholinergic motoneurons and blocks the release of the neurotransmitter
acetylcholine. As a consequence the striated muscles are paralysed ultimately resulting in the deadly paralysis of the diaphragm. Only mechanical ventilation at intensive care
units and the rapid administration of equine antitoxin (trivalent
antitoxin licensed in the EU) within the first 48 h after symptoms like difficult swallowing or speaking, facial weakness on both sides of the face, blurred vision, drooping
eyelids, trouble breathing, nausea, vomiting and abdominal cramps (only in food-borne botulism) occurred, will prevent death of the patient. The EU/EEA notification rate of
botulism for 2014 was 0.02 cases per 100.000 population comprising 123 reported and 91 lab confirmed cases of botulism (data from IT and LI lacking). The most affected age groups
were 0-4 and 45-65 year old males, and 0-4 and 25-44 year old females. Age group 0-4 comprises a high proportion of infant botulism (ECDC 2016).
Structure of BoNT
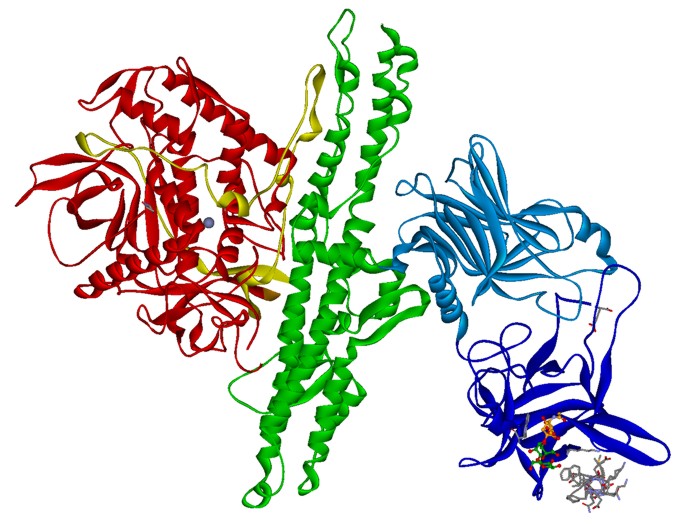 Fig. 1. Crystal structure of BoNT/B (PDB: 1F31 & 2NM1) with bound
sialyllactose (ball-stick) and synaptotagmin-II peptide (gray sticks). HC fragment is shown in blue, HN domain in green and LC in red ribbon.
Fig. 1. Crystal structure of BoNT/B (PDB: 1F31 & 2NM1) with bound
sialyllactose (ball-stick) and synaptotagmin-II peptide (gray sticks). HC fragment is shown in blue, HN domain in green and LC in red ribbon.The BoNT are classical A-B protein toxins like ricin or diphtheria toxin and
consist of an enzymatically active 50 kDa A-domain called light chain (LC) and a 100 kDa B-domain called heavy chain (HC) which are covalently linked by a disulfide bridge (Fig.
1). The HC consists of two domains, the amino-terminal half HN is responsible for the translocation of the LC into the neuronal cytosol (Montal 2010) whereas the carboxyl-terminal half HC mediates the highly
neurospecific binding to its cellular receptors (Binz & Rummel 2009) thereby
exerting its extraordinary toxicity.
Mechanism of action
Once the BoNT has reached the circulation it accumulates via interaction with polysialylated glycolipids (gangliosides) on the surface of
motoneurons. Subsequent binding to a synaptic vesicle protein like SV2 or synaptagmin leads to its uptake. Acidification of the synaptic vesicle allows translocation and release
of the LC into the cytosol where it halts the core machinery for neurotransmitter release by cleaving one of the three SNARE proteins SNAP-25, synaptobrevin/VAMP (vesicle
associated membrane protein)-2 and syntaxin-1.
Detection
Detection of BoNT at relevant concentrations is challenging because BoNT is extremely lethal and therefore a test must be correspondingly
sensitive. The currently accepted test for functional detection of BoNT is still the standard mouse bioassay (Sesardic et al., 2003). According to DIN 10102, 14 mice in seven groups are each intraperitoneally injected with 0.4-0.5 mL of a filter sterilized sample, and
watched for signs of intoxication. BoNT intoxicated mice will usually die within 6-96 hours, depending on the level of toxin in the sample. But the mouse bioassay is cruel, time
consuming, costly, impractical for screening large numbers of samples and cannot be used in the field. A fully functional highly sensitive replacement assay is the mouse phrenic
nerve hemidiaphragm assay recommended as method for detecting BoNT (Bigalke & Rummel, 2015, toxogen GmbH). In addition for a rapid and sensitive assay for BoNT, in vitro assays have been under development in
recent years (Dorner et al., 2013). Assays that detect BoNT proteolytic activity
employ naturally occurring or synthetic analogues of SNARE proteins combined with methods for detecting the cleaved products like FRET or MS.
References
Bigalke, H., Rummel, A. (2015) Botulinum Neurotoxins: Qualitative and Quantitative Analysis
Using the Mouse Phrenic Nerve Hemidiaphragm Assay (MPN). Toxins (Basel), 7(12), 4895-905.
Binz, T. and Rummel, A. (2009) Cell entry strategy of clostridial neurotoxins. J Neurochem, 109, 1584-1595.
Dorner, M.B., Schulz, K.M., Kull, S., Dorner, B.G. (2013) Complexity of botulinum neurotoxins: challenges for detection technology. Curr Top Microbiol Immunol, 364, 219-55.
European Centre for Disease Prevention and Control (2011) Annual Epidemiological Report 2011. Reporting on 2009 surveillance data and 2010 epidemic intelligence data, 65-67.
Gu, S., Rumpel, S., Zhou, J., Strotmeier, J., Bigalke, H., Perry, K., Shoemaker, C. B., Rummel, A. and Jin, R. (2012) Botulinum neurotoxin is shielded by NTNHA in an interlocked complex. Science, 335, 977-981.
Lee, K., Gu, S., Jin, L., Le, T.T., Cheng, L.W., Strotmeier, J., Kruel, AM., Yao, G., Perry, K., Rummel, A., Jin, R. (2013) Structure of a bimodular botulinum neurotoxin complex provides insights into its oral toxicity. PLoS Pathog, 9(10).
Lee, K., Zhong, X., Gu, S., Kruel, AM., Dorner, M.B., Perry, K., Rummel, A., Dong, M., Jin, R. (2014) Molecular basis for disruption of E-cadherin adhesion by botulinum neurotoxin A complex. Science, 344(6190), 1405-10.
Matsumura, T., Sugawara, Y., Yutani, M., Amatsu, S., Yagita, H., Kohda, T., Fukuoka, S., Nakamura, Y., Fukuda, S., Hase, K., Ohno, H., Fujinaga, Y. (2015) Botulinum toxin A complex exploits intestinal M cells to enter the host and exert neurotoxicity. Nat Commun, 6:6255.
Montal, M. (2010) Botulinum neurotoxin: a marvel of protein design. Annu Rev Biochem, 79, 591-617.
Sesardic, D., Leung, T. and Gaines Das, R. (2003) Role for standards in assays of botulinum toxins: international collaborative study of three preparations of botulinum type A toxin. Biologicals, 31, 265-276.
Binz, T. and Rummel, A. (2009) Cell entry strategy of clostridial neurotoxins. J Neurochem, 109, 1584-1595.
Dorner, M.B., Schulz, K.M., Kull, S., Dorner, B.G. (2013) Complexity of botulinum neurotoxins: challenges for detection technology. Curr Top Microbiol Immunol, 364, 219-55.
European Centre for Disease Prevention and Control (2011) Annual Epidemiological Report 2011. Reporting on 2009 surveillance data and 2010 epidemic intelligence data, 65-67.
Gu, S., Rumpel, S., Zhou, J., Strotmeier, J., Bigalke, H., Perry, K., Shoemaker, C. B., Rummel, A. and Jin, R. (2012) Botulinum neurotoxin is shielded by NTNHA in an interlocked complex. Science, 335, 977-981.
Lee, K., Gu, S., Jin, L., Le, T.T., Cheng, L.W., Strotmeier, J., Kruel, AM., Yao, G., Perry, K., Rummel, A., Jin, R. (2013) Structure of a bimodular botulinum neurotoxin complex provides insights into its oral toxicity. PLoS Pathog, 9(10).
Lee, K., Zhong, X., Gu, S., Kruel, AM., Dorner, M.B., Perry, K., Rummel, A., Dong, M., Jin, R. (2014) Molecular basis for disruption of E-cadherin adhesion by botulinum neurotoxin A complex. Science, 344(6190), 1405-10.
Matsumura, T., Sugawara, Y., Yutani, M., Amatsu, S., Yagita, H., Kohda, T., Fukuoka, S., Nakamura, Y., Fukuda, S., Hase, K., Ohno, H., Fujinaga, Y. (2015) Botulinum toxin A complex exploits intestinal M cells to enter the host and exert neurotoxicity. Nat Commun, 6:6255.
Montal, M. (2010) Botulinum neurotoxin: a marvel of protein design. Annu Rev Biochem, 79, 591-617.
Sesardic, D., Leung, T. and Gaines Das, R. (2003) Role for standards in assays of botulinum toxins: international collaborative study of three preparations of botulinum type A toxin. Biologicals, 31, 265-276.